Fda Booster - Pfizer And Biontech Submit Early Data To Fda For Booster Covid 19 Shot
September 18 2021 617 AM 4 min read. A panel advising the US Food and Drug Administration FDA has recommended boosters of Pfizers Covid-19 vaccine for people 65 and over and those at high risk.
FDA committee says Pfizer-BioNTech boosters should be targeted citing lack of data for people under 65.
Fda booster. Robert Summa Appeal-Democrat Marysville Calif. 17 2021 at 458 pm. FDA panel approves Pfizer boosters for some 0329.
But it voted against recommending a. The FDA released the findings Wednesday in a report analyzing data submitted by Pfizer Inc. Doran Fink told the meeting the agency was not sure either.
We can tweak this as need be Dr. Marion Gruber director of the FDAs Office of Vaccines Research Review and deputy director Phil Krause will exit the agency in October and November respectively according to a letter shared. Pfizer boosters get OK from FDA panel for people over 65 at high risk.
A Food and Drug Administration advisory panel overwhelmingly voted Friday against giving Pfizer-BioNTechs Covid-19 booster. FDA staff declines to take stance on Pfizers Covid vaccine booster shots citing lack of verified data Published Wed Sep 15 2021 1122 AM EDT Updated Wed Sep 15 2021 833 PM EDT Berkeley. And BioNTech SE as part of their request for authorization for their vaccine to be given as a booster.
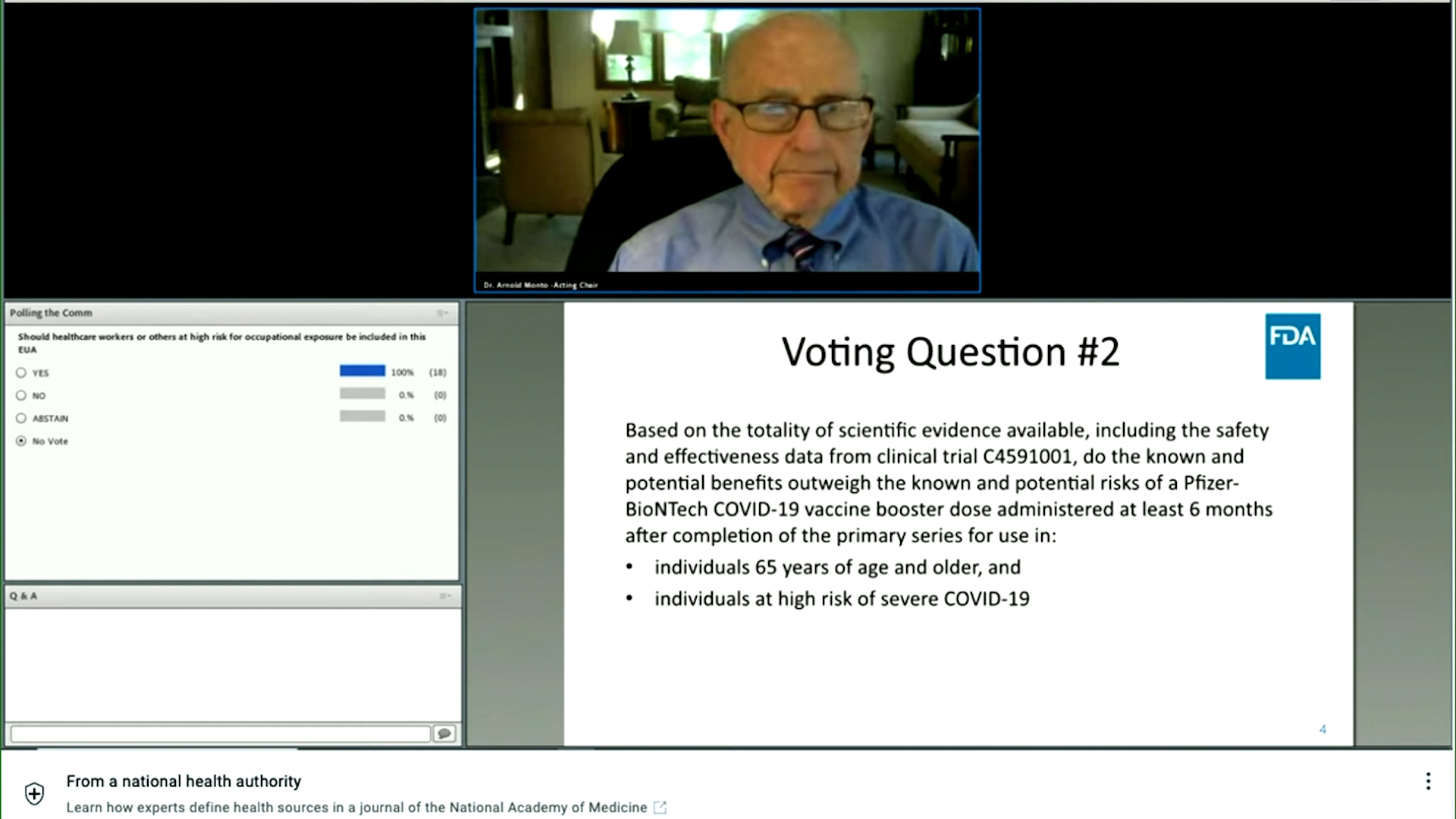
FDA announced a virtual VRBPAC meeting to discuss the Pfizer-BioNTech supplemental application for a third booster dose of Comirnaty COVID-19 Vaccine mRNA. When an FDA advisory panel agreed Friday that only those 65 and older or otherwise at high risk of severe COVID-19 should get vaccine booster shots to bolster their immunity Colleen Logan found. Closing Bell We are not bound at FDA by your vote just so you understand that.
FDA panel votes for Pfizer booster for those 65 and at higher risk. FDA committee votes to authorize booster shots for 65-plus high-risk. Krause questioned whether the sharp drop in effectiveness was an artifact of the statistical analysis used by the authors and not a real reflection of COVID-19 case numbers.
Marion Gruber director of the FDAs Office of Vaccines Research Review and deputy director Phil Krause are set to leave the agency this fall with sources telling Politico that the two. 17 2021 at 346 pm. Fri 17 Sep 2021 1221 EDT.
The FDAs Dr. We really dont have enough data yet to know what the risk of myocarditis or pericarditis would be following a booster. A panel of outside experts for the Food and Drug Administration have unanimously recommended an emergency use authorization for a third dose of.
FDA panel recommends Pfizer boosters for people 65 and older or high risk 39 minutes ago NYC hostess assaulted after asking Texas patrons for proof of COVID-19 vaccination. Booster blast Tension over boosters rises as FDA regulators quit and publicly blast Bidens plan Top FDA regulators side with WHO on boosters citing insufficient data. But FDAs Phil Krause one of the FDA officials who announced he was resigning reportedly over Bidens booster plan raised questions about the Kaiser Permanente study at Fridays meeting.
FDA panel recommends Pfizers COVID-19 booster for 65 and older but not for the general public Last Updated. Food and Drug Administration FDA advisory panel on Friday endorsed emergency approval for the Pfizer-BioNTech COVID-19 vaccine booster shot at least six months following the second dose. Scientific advisers to the Food and Drug Administration FDA will debate the safety and efficacy of recommending a booster shot for fully vaccinated Americans who.
FDA advisory panel votes against vaccine booster for most Americans.

Fda Scientists Skeptical Of Covid 19 Booster Evidence Ahead Of Key Meeting Thehill

Fda Panel Issues Narrow Recommendation For Covid Vaccine Booster Doses

Pfizer And Biontech Submit Early Data To Fda For Booster Covid 19 Shot

Coronavirus Updates Fda Withholds Booster Recommendation Outside Panel To Examine Friday Abc7 New York